Question Number: 138
PDR Number: SQ22-000159
Date Submitted: 24/02/2022
Department or Body: Department of Health
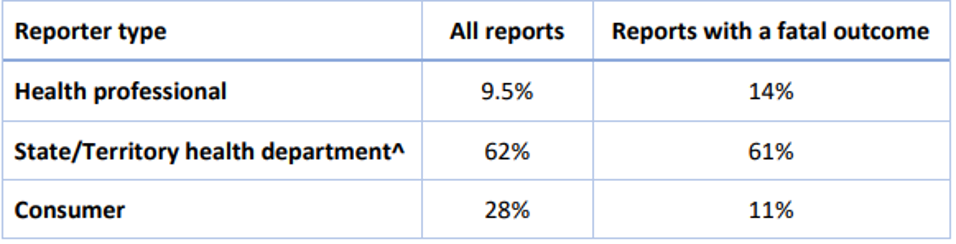
This provides information on the reporter type for adverse event reports for COVID-19 vaccines received up to 6 March 2022: All adverse event reports, including those with a fatal outcome, remain in the Therapeutic Goods Administration (TGA) database even if they have not been assessed as vaccine related so that they are included in analyses to detect safety signals based on patterns of reporting. The TGA encourages consumers and health professionals to report suspected side effects, even if there is only a very small chance a vaccine was the cause. Health professionals in New South Wales, Western Australia, Queensland, Northern Territory and Australian Capital Territory are required under public health legislation to notify adverse events following immunisation to their state or territory health department. These reports are then submitted to the TGA. Many suspected adverse events may be coincidental, rather than being caused by the vaccine. As the number of people being vaccinated has increased, so has reporting of adverse events, including fatal events, with a temporal association with vaccination. All deaths reported to the TGA in people who have been recently vaccinated against COVID-19 are assessed to determine whether the information provided suggests a possible link between vaccination and the causes of death, or if further information is required to make an assessment. These case reviews consider the strength of the evidence available to determine whether the clinical conditions which led to a fatal outcome represent an emerging safety signal for the vaccine. To 6 March 2022, 11 fatal adverse events have been assessed as being related to vaccination.