Question Number: 248
PDR Number: SQ22-000618
Date Submitted: 21/11/2022
Department or Body: Department of Health
Question 253 A full list of protocol deviations on or prior to seven days after dose two can be found in Table 1. These include dosing and administration errors. It is important to note that these did not include vaccine injuries. The table below shows characteristics of subjects excluded from evaluable efficacy population due to important protocol deviations – on or prior to seven days after dose two. (See first table) Question 254
First, the table on page 33 of FOI 2389 -1
(available at: www.tga.gov.au/sites/default/files/foi-2389-01.pdf) shows that the 1,790
participants who were excluded from the evaluable efficacy population on days 7 and 14
had received the vaccine. This is compared to 1,584 and 1,585 participants in the placebo
group who were excluded from the evaluable efficacy population on days 7 and 14.
Second, as listed in Table 1, page 33 of FOI 2389-1, reasons participants were excluded from
the evaluable efficacy population include:
randomised but did not meet all eligibility criteria
did not provide informed consent
did not receive all vaccinations as randomised or did not receive dose two within the
predefined window (19-42 days after dose one)
had other important protocol deviations on or prior to seven days after dose two,
and/or
had other important protocol deviations on or prior to 14 days after dose two. Question 255
Assuming a true Vaccine Efficacy of 60 per cent after the second dose of study intervention,
a total of approximately 164 first-confirmed COVID-19 illness cases would provide
approximately 90% power. This should be achieved with 17,600 evaluable participants per
group, or 21,999 vaccine recipients randomised in a 1:1 ratio with placebo.
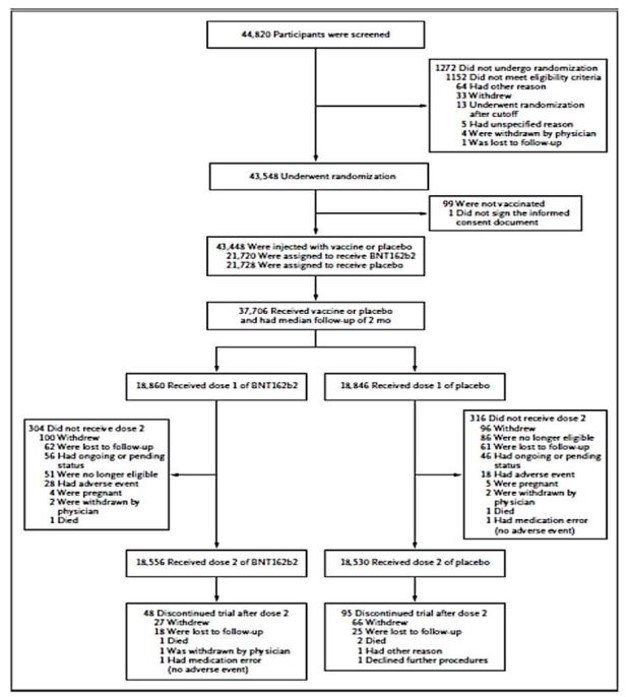
Based on this, 21,000 participants were assigned to each group, but not all of them were
vaccinated. Only approximately 18,850 participants were vaccinated and efficacy was
evaluated for the vaccinated subjects only. The participant flow is detailed below: (See second table). Question 256
Most participants who were excluded from the evaluable efficacy population had not
received all vaccinations as randomised or did not receive dose two within the predefined
window (i.e. 19 to 42 days after dose one). The exact number of subjects or further
subgroup details are not available.
There were 311 participants (1.4 per cent) in the vaccinated group and 60 participants
(0.3%) in the placebo group excluded for having important protocol deviations on or prior to
seven days after Dose two. Investigational Product related errors or deviation were noted
for 263 subjects. Further subgrouping or patient level data were not provided to nor
requested by the Therapeutic Goods Administration.
A similar number of subjects did not receive their second dose in the placebo group.
Since it was balanced between both groups (vaccine and placebo), the efficacy outcome of
the study was not affected. (See link for tables)