Question Number: 120
PDR Number: SQ22-000152
Date Submitted: 24/02/2022
Department or Body: Department of Health
159. Selected categories of adverse event report that do not contribute to our safety
monitoring of medicines and vaccines approved for use in Australia are excluded from the DAEN. Collectively, these make up a very small proportion of all adverse event reports to the Therapeutic Goods Administration (TGA). They are:
- Reports that are not valid (the criteria for valid adverse event reports are included in the response to question 187 in SQ22-000157)
- Reports that are withdrawn or that are duplicates (as described in the response to question 187 in SQ22-000157), and
- Reports for medicines and vaccines accessed via the Special Access Scheme,
Authorised Prescriber scheme, clinical trial notification scheme or clinical trial
exemption scheme are not included, except where the adverse event report
also includes a suspected general marketed medicine or vaccine.
160. Yes. The TGAs internal database, known as the Adverse Event Management System (AEMS), is a separate database which contains extensive information on all medicine and vaccine adverse events reported to the TGA.
161. Data fields to be displayed in the DAEN are transferred to an intermediary database which is accessed via the DAEN search web interface. This process is automated and transfers the data fields included in the DAEN, with some logic steps applied to improve data quality. For example, if the same medicine or vaccine is entered twice in the same report, it will only appear once in the medicines reported as taken in the DAEN. For adverse event data to appear in the DAEN the case needs to:
- be accepted (e.g., not rejected as a duplicate report)
- not be identified as involving a medicine or vaccine accessed via the Special Access Scheme, Authorised Prescriber scheme, clinical trial notification scheme
or clinical trial exemption scheme, and - have at least one medicine or vaccine identified as being suspected of causing
the adverse event (either directly or through an interaction), and - have a report date of more than 14 days ago.
162. 98.3 per cent of reports received by the TGA for COVID-19 vaccines are included in the DAEN, as summarised in the table below.
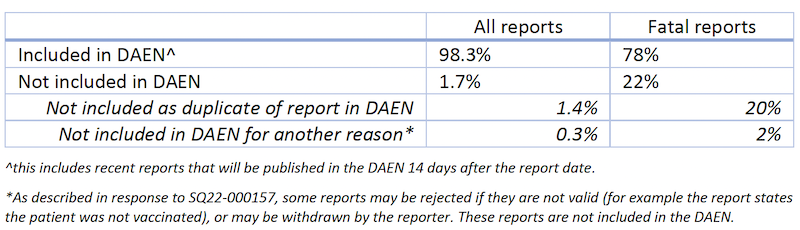
163. The higher proportion of fatal reports not included in the DAEN is due to the TGA receiving and identifying a higher number of duplicate reports. This is in part due to the higher scrutiny of reports of death related in time to receipt of a vaccination.
206. Questions regarding any adverse event databases held by state and territory health departments should be directed to these departments. The TGA has two main
databases which hold adverse event; AEMS and the database that stores the DAEN
data. In addition, AEMS data is transferred each night to a data store which supports
signal detection and investigation and our data visualisation platform.
207. As stated in response to SQ22-000140, the TGA has well-established processes for sharing reports of adverse events following immunisation (AEFI) with all state and
territory health departments. We have confidence in the robust information sharing
processes that are in place, in particular for all AEFI that may have regulatory
significance. Questions regarding any adverse event data not shared with the TGA
should be directed to state and territory health departments.
208. The TGA cannot comment on data not reported to us by state and territory health departments. However, the TGA has received over 70,000 reports from state and territory health departments for COVID-19 vaccines, with over 98.7 per cent of these reports included in the DAEN.








