Question Number: 162
PDR Number: SQ22-000112
Date Submitted: 24/02/2022
Department or Body: Department of Health
Question 43
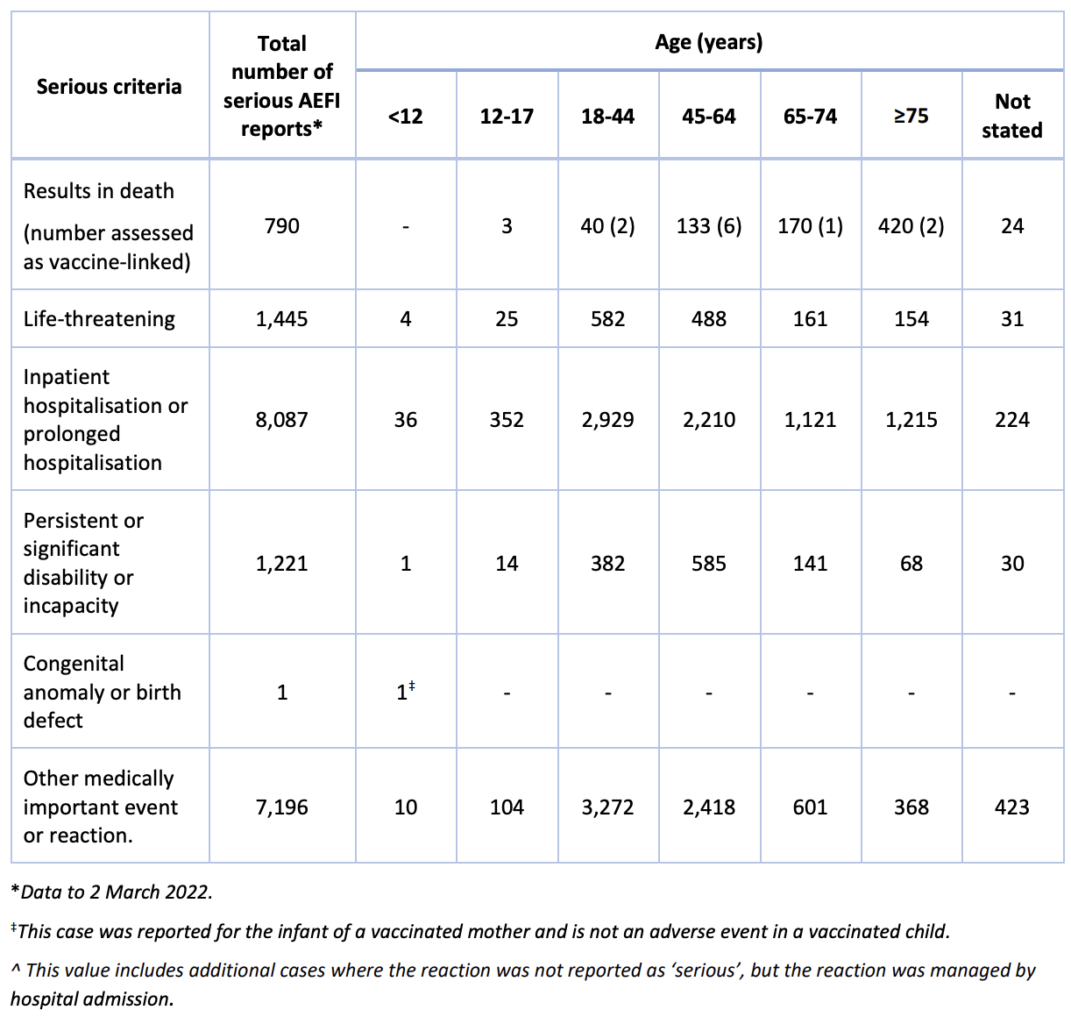
The number of reports to 2 March 2022 for different age groups in each serious category
are provided in the following table.
Although the option to classify a reaction as serious is available to reporters, this
assessment reflects the reporters view of seriousness, and does not mean that the
Therapeutic Goods Administration (TGA) has confirmed that the report meets the serious
criteria. Some reports classified as serious may represent common and expected reactions,
such as injection site pain, fever, headache and/or nausea.
Question 44
The TGA does not intend to publish the number of serious adverse event reports stratified by age and serious criteria in the weekly safety report. Simple publication of the number and type of adverse event reports received by the TGA but not reviewed is not a valid method of assessing vaccine safety. Adverse event data is used to identify safety signals.
The TGA provides an analysis and summary of these data and the outcomes of its
investigations of safety issues, rather than raw adverse event report data, in the weekly COVID-19 vaccine safety report. The TGA also publishes adverse event information in the database of Adverse Event Notifications (DAEN).








